Quest Diagnostics Covid 19 Swab Test - Covid-19 Realtime Info
Contact your doctor or another authorized healthcare provider to discuss where in your community to get tested for an active infection.

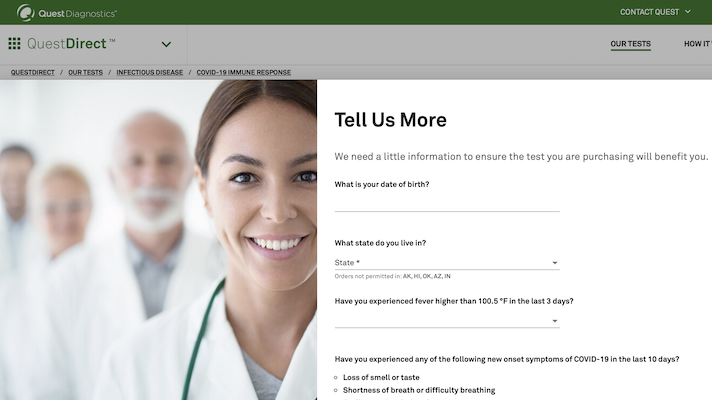
Quest diagnostics covid 19 swab test. 360bbb 3b1 unless the authorization is terminated or revoked sooner. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical andor. Quest quest diagnostics the associated logo nichols institute and all associated quest diagnostics. Get easy to understand results through your secure myquest account.
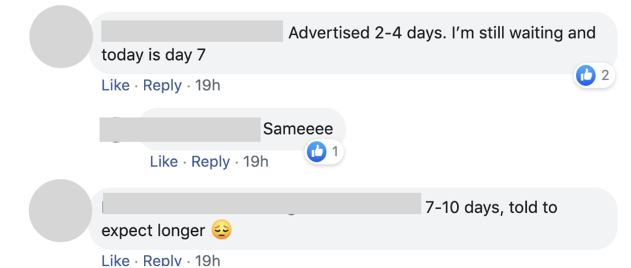
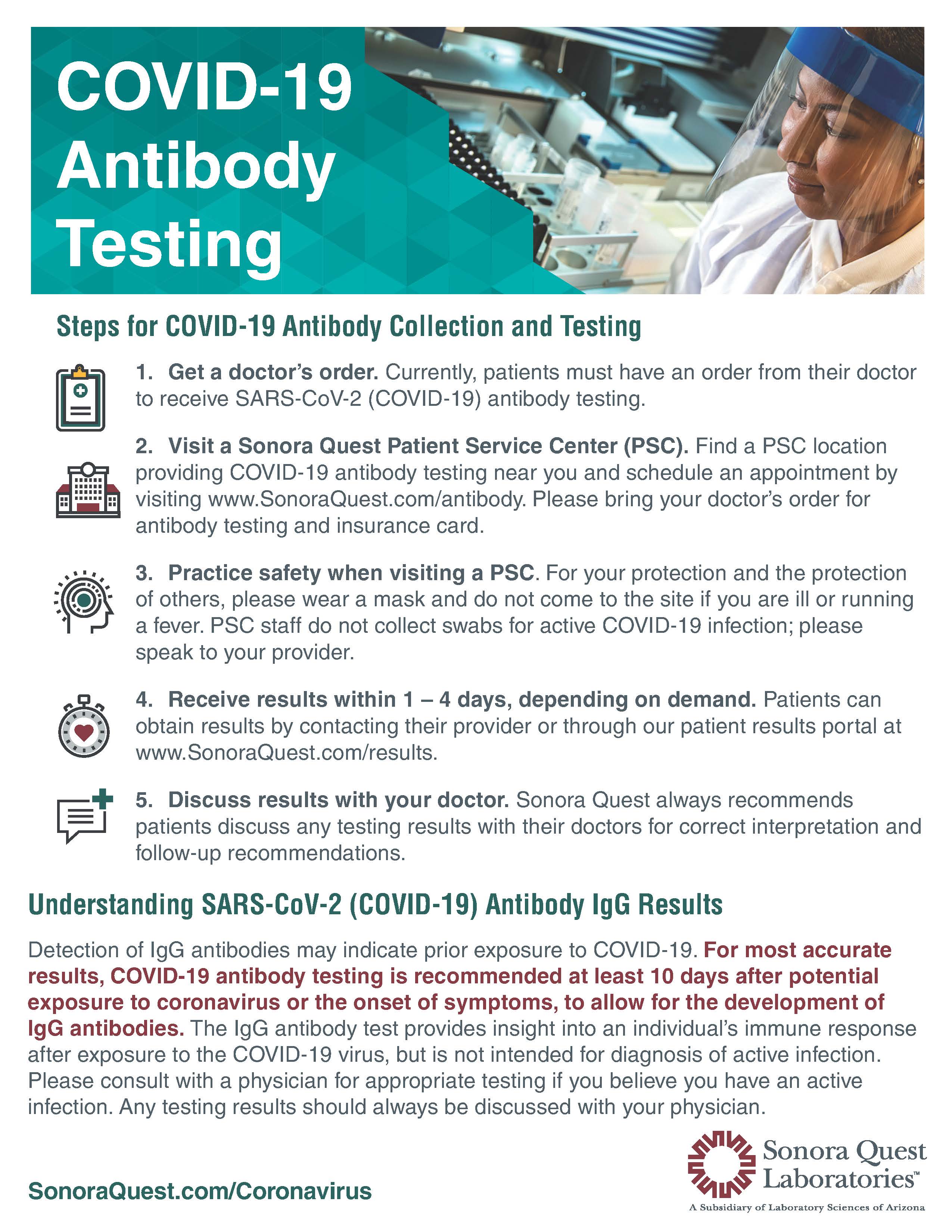
Covid 19 testing is not available in our patient service centers. Quest diagnostics is a leader in infectious disease testing services with a broad menu of molecular antibody and other test services to aid. All tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection andor diagnosis of covid 19 under section 564b1 of the act 21 usc. April 20 2020 quest launched igg antibody serology testing by blood draw to help patients and healthcare professionals identify who may have recovered from a prior infection and may possibly have a lower risk of reinfection.
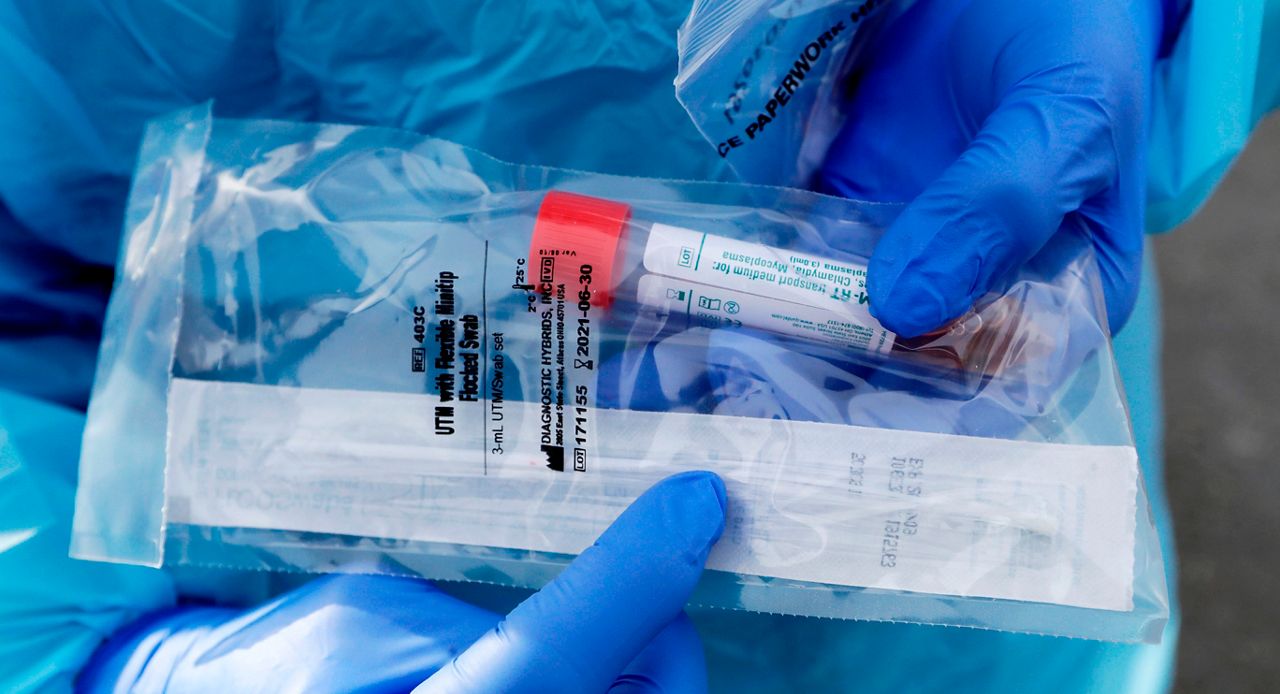
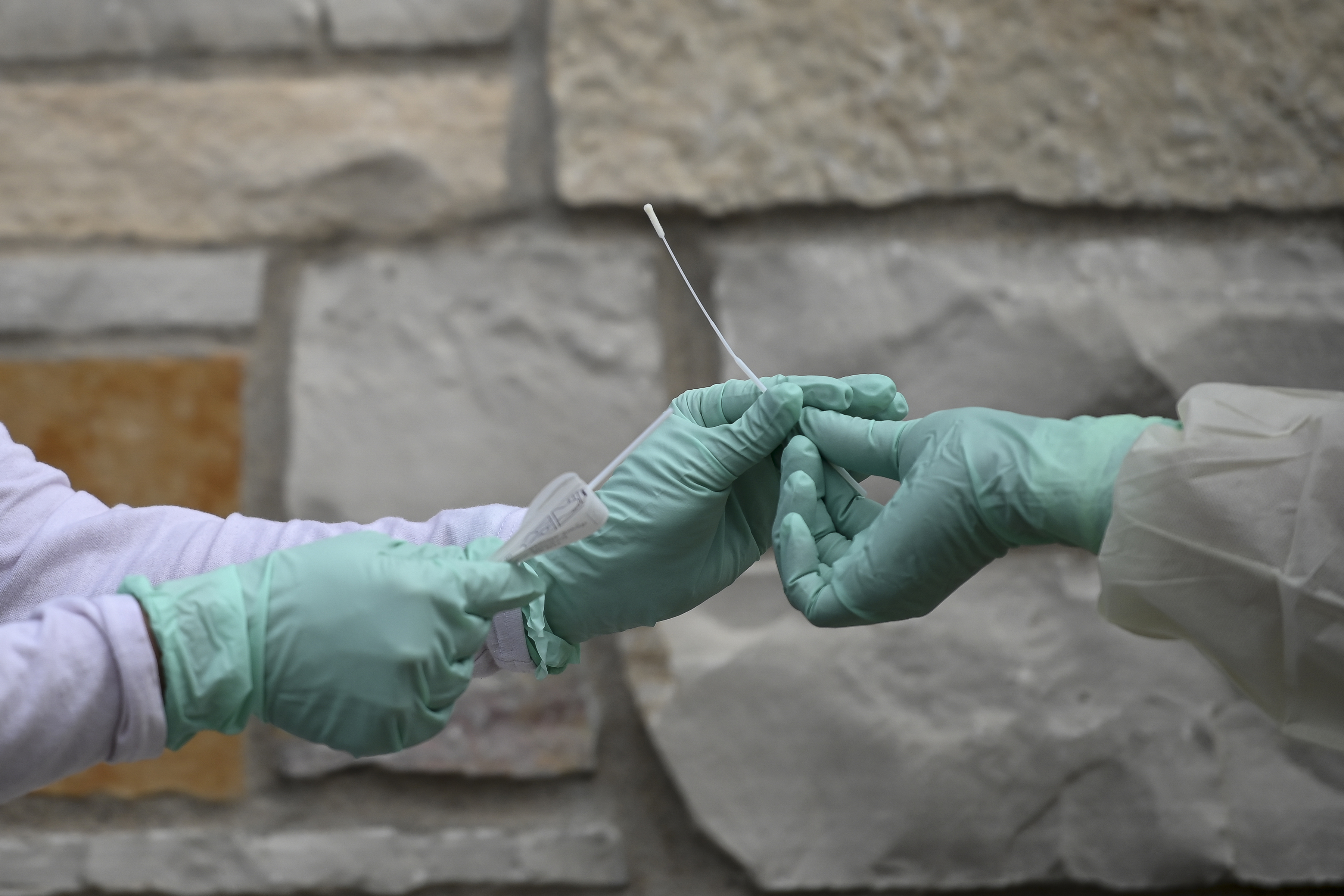
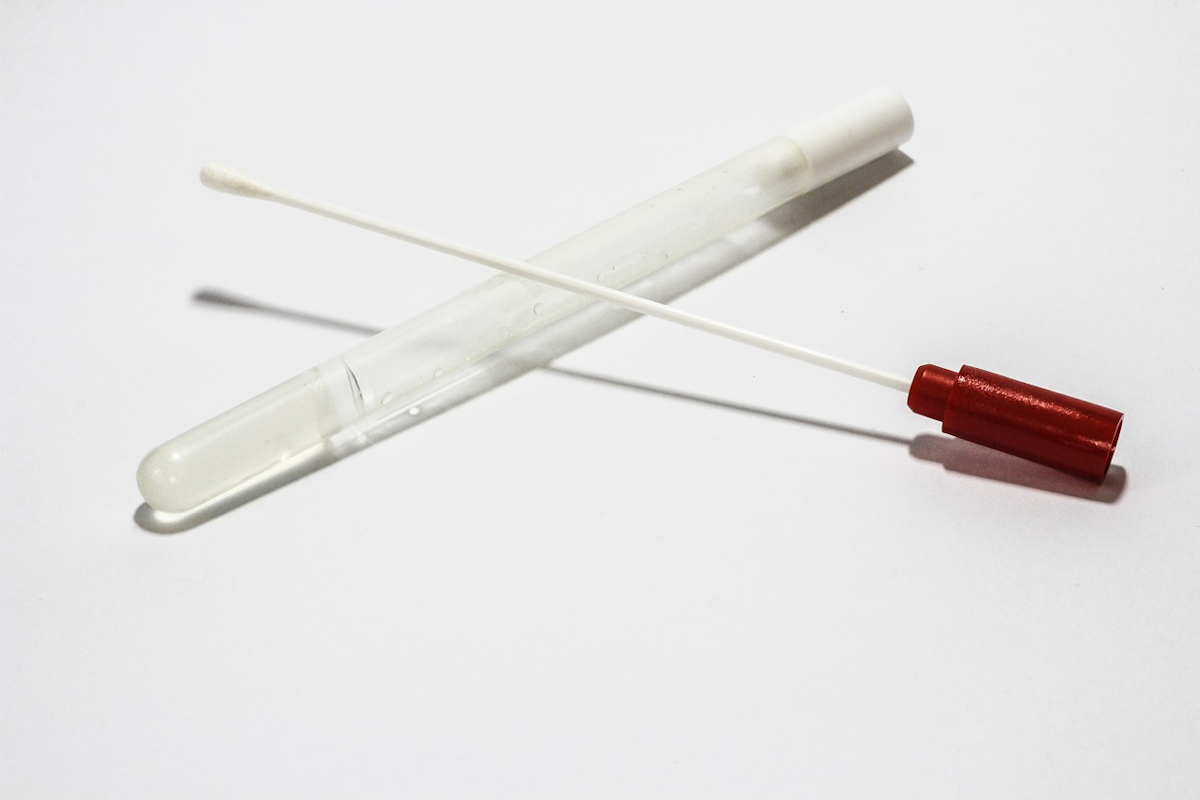
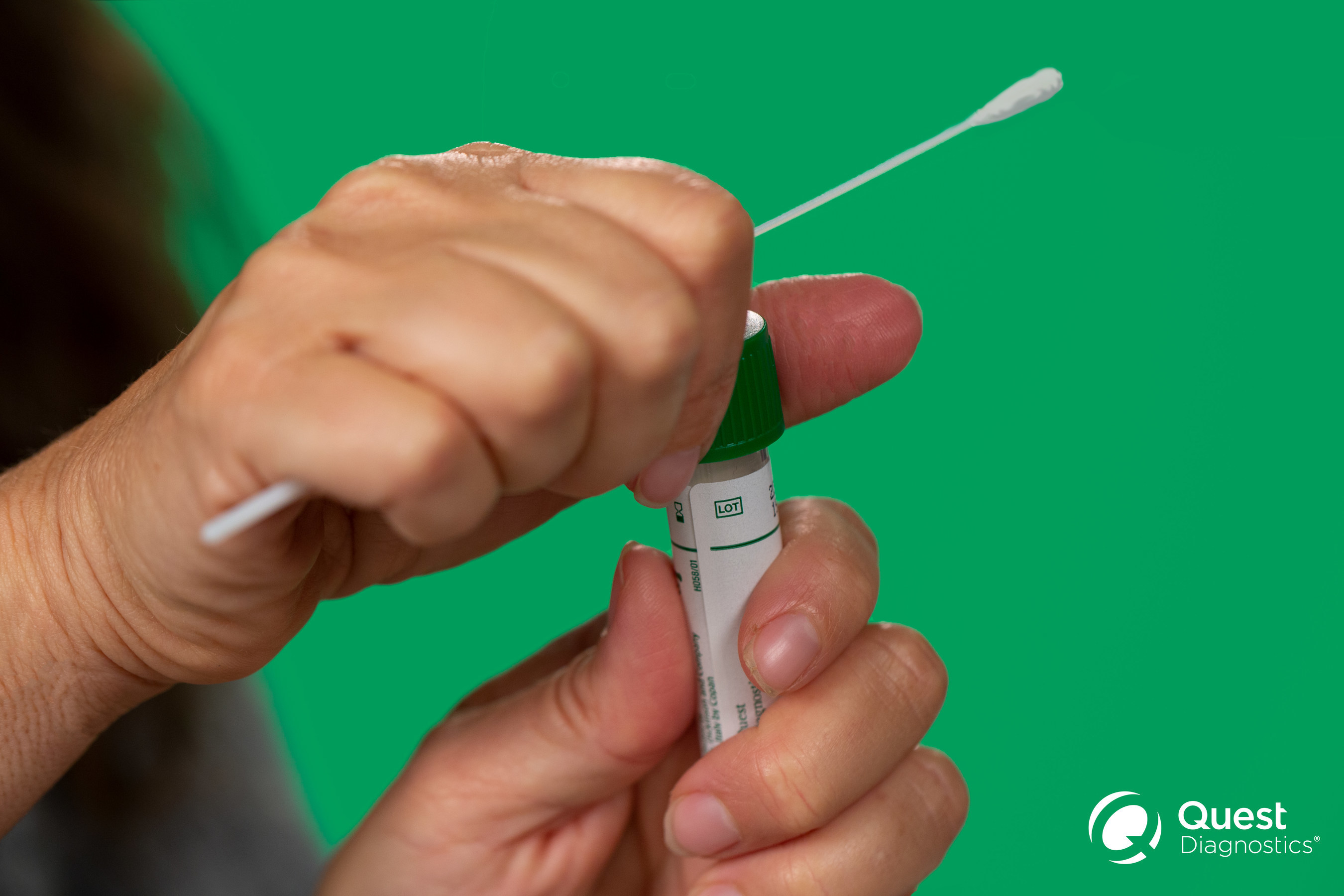
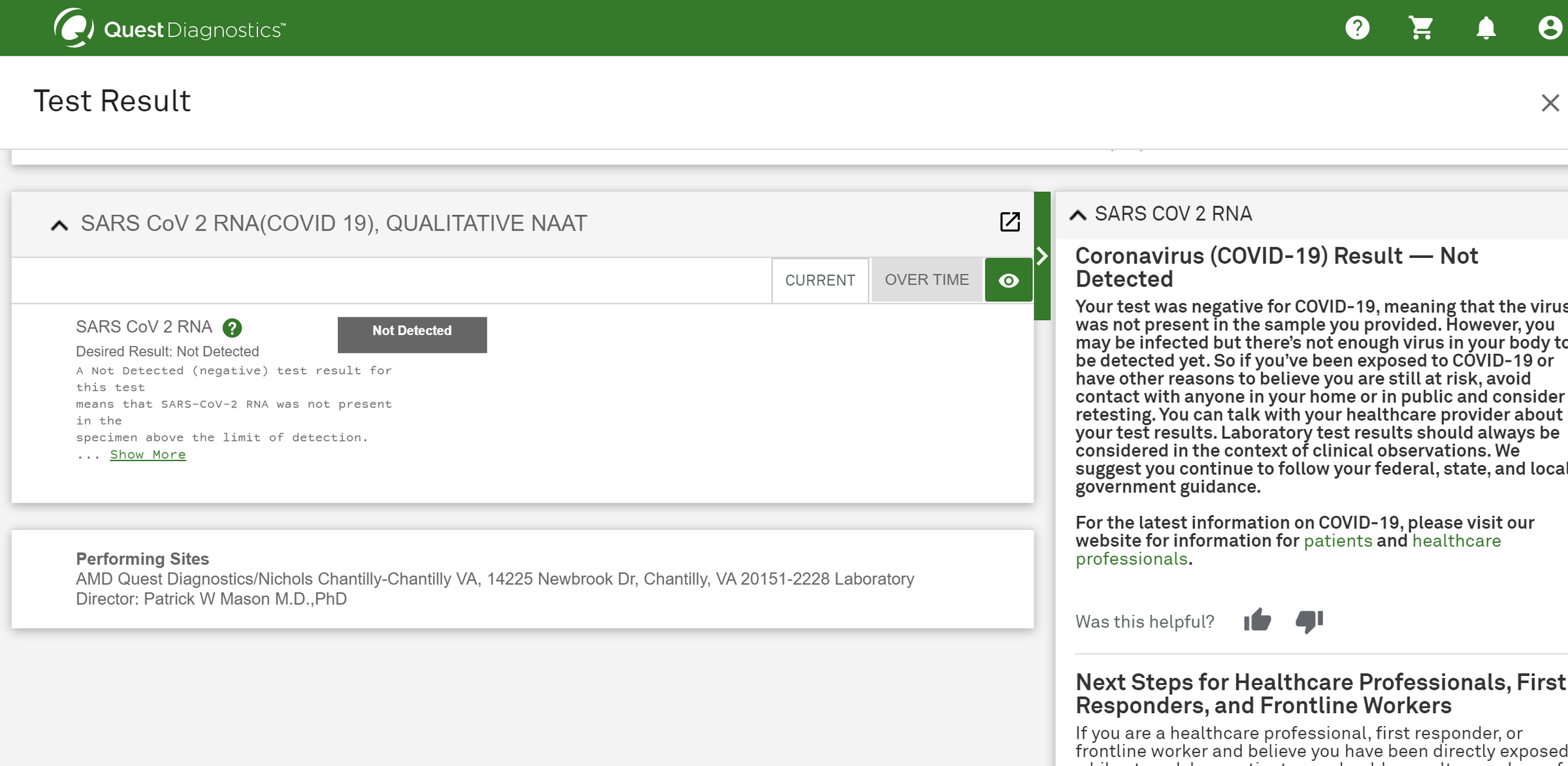
A healthcare professional will provide guidance as you swab your nasal passage. Through qualitative multi target molecular diagnostics this testing option helps to detect the presence of sars cov 2. Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19. Quest diagnostics is receiving covid 19 specimens and performing testing nationwide.
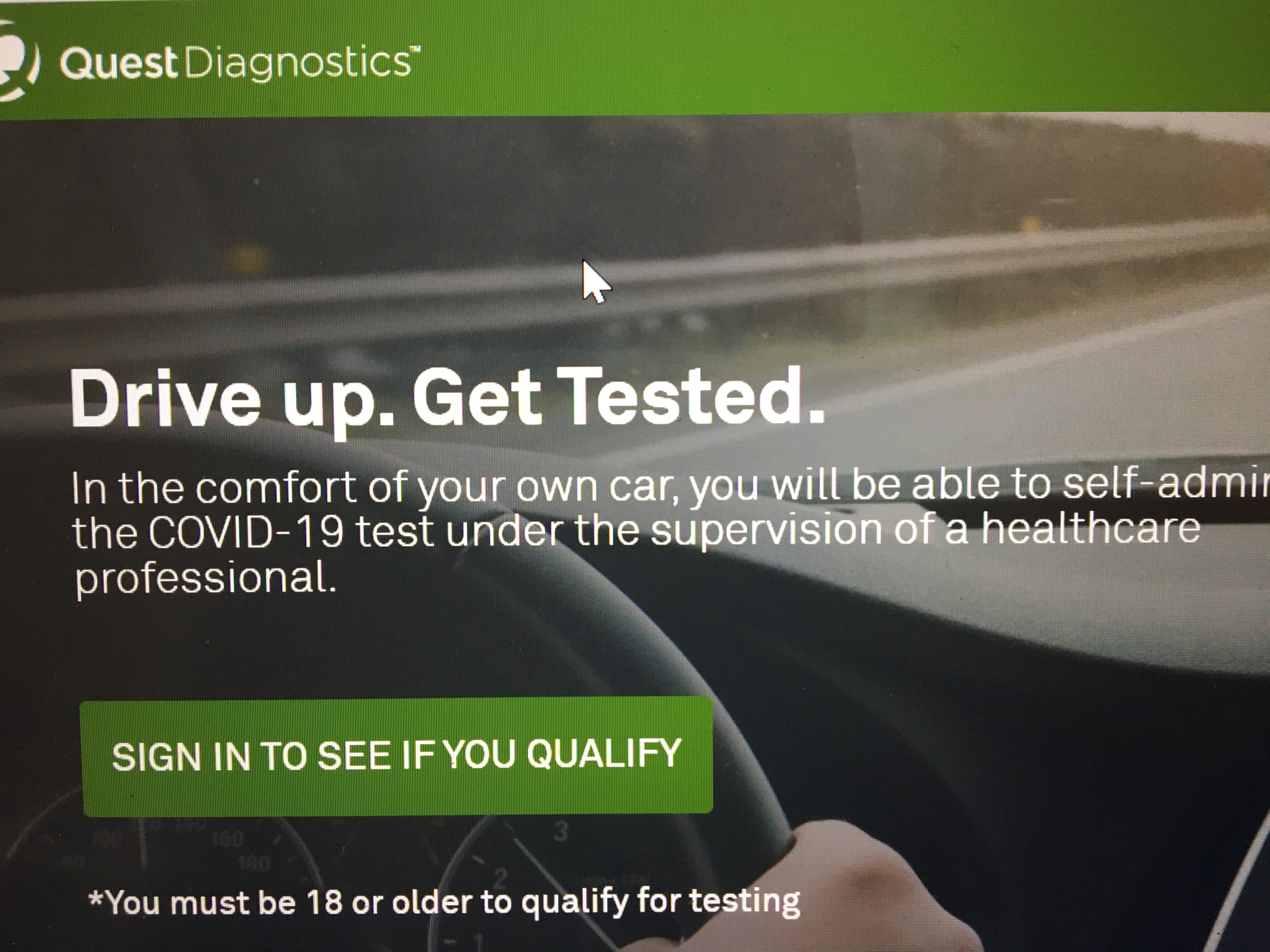
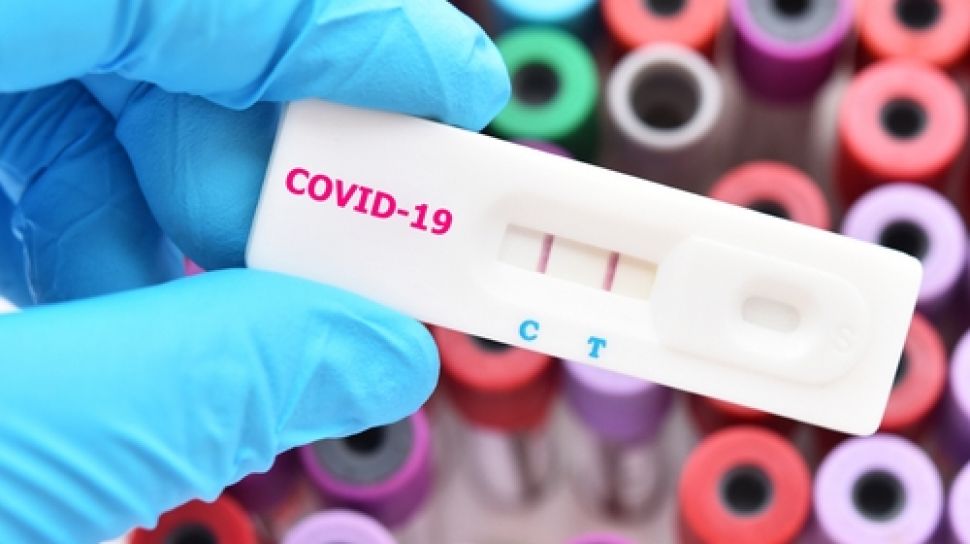
Quest quest diagnostics any associated logos and all associated quest diagnostics registered or unregistered trademarks. A molecular test test code 39448 is available to test symptomatic patients for covid 19. Quest and walmart are providing drive thru covid 19 self swab tests at select locations across the country. Quest introduced a new lab developed test for covid 192 days before the virus was labeled a worldwide pandemic.
360bbb 3b1 unless the authorization is terminated or revoked sooner. Find a drive thru event option 2. The swab should be inserted about half. Covid 19 testing with sars cov 2 rna covid 19 qualitative naat test code 39448 for specimen collection by a healthcare professional.
Coronavirus disease 2019 or covid 19 formally known as 2019 ncov is the name for the respiratory syndrome caused by infection with severe acute respiratory syndrome coronavirus 2 sars cov 2. All tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection andor diagnosis of covid 19 under section 564b1 of the act 21 usc.















































:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/arc-anglerfish-arc2-prod-gmg.s3.amazonaws.com/public/7EFBIC7AIFHEVP46FD4OLHJE5U.jpg)